OAT3 (SLC22A8)
➦ The Role of OAT3 in the Kidney and Regulatory Considerations for New Drugs
The human organic anion transporter OAT3 (SLC22A8) is highly expressed at the basolateral membrane of proximal tubule cells in human kidneys, where it, like OAT1, mediates the initial uptake for renal secretion of a wide range of substrates. OAT3 preferentially transports conjugated endogenous steroid hormones as well as numerous exogenous compounds, including antibiotics, diuretics, NSAIDs, histamine-receptor 2 blockers, antivirals, cytostatics, and toxins. Its activity strongly influences drug clearance, systemic exposure, and the potential for nephrotoxicity or drug–drug interactions.
Recognizing its importance, regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) recommend evaluating new molecular entities for their interaction with OAT3 during preclinical and clinical development. These assessments help predict renal safety, optimize dosing, and identify possible drug–drug interactions, making OAT3 studies a standard component of drug development programs targeting renal excretion.
| Main localization: | Kidney |
| Transporter assay: | Uptake transporter assay (potential inhibitors or substrates) |
| Probe substrates: | Estrone-3-sulfate (ES) |
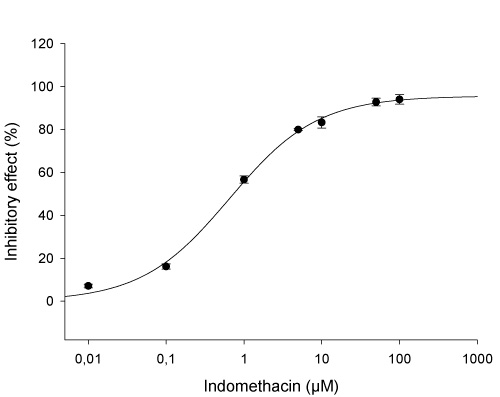
| Probe inhibitors: | Indomethacin, probenecid |
| Regulatory relevance: | FDA and EMA guidance |
| Important interacting drugs: | Methotrexate, ochratoxin A, olmesartan, pitavastatin, pravastatin, rosuvastatin, probenecid, telmisartan, olmesartan, sulfasalazine, quinapril, ketoprofen, Losartan, furosemide, ibuprofen, indomethacin, bumetanide |
| From other species: | rOat3, mOat3 |
➦ Concentration dependent inhibition of human OAT3-mediated Estrone-3-sulfate (ES) uptake by the probe inhibitor indomethacin